Link to publication:
https://www.sciencedirect.com/science/article/abs/pii/S0969212623002897?dgcid=author
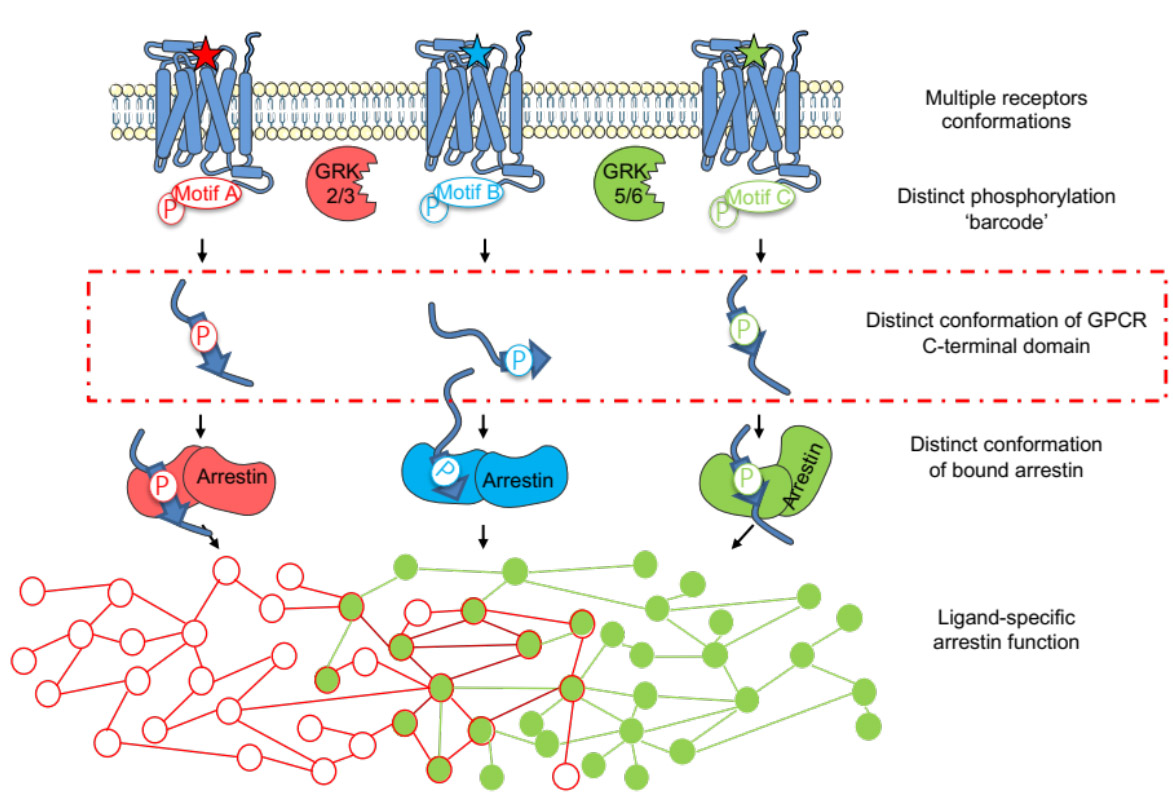
The signaling pathways of arrestin-dependent G protein-coupled receptors (GPCRs) are regulated by the phosphorylation state of its C-terminal domain. The molecular basis of the arrestin-receptor interaction is not fully understood. In this study, we investigated the impact of phosphorylation on the conformation of the C-terminal region of three rhodopsin-like GPCR (V2R, GHSR, and β2AR). Using phosphomimetic models, the team of Nathalie Sibille, in collaboration with the team of Jean-Louis Banères (IBMM), has identified pre-formed secondary structure elements, or short linear motifs (SLiMs), that undergo specific conformational transitions upon phosphorylation. It's important to note that such conformational transitions occur in the region interacting with arrestin-2. Thus, our results suggest a model in which the phosphorylation-dependent conformation of the C-terminal regions of GPCRs would modulate arrestin binding and consequently the signaling of arrestin-dependent pathways. This illuminates how signaling through GPCRs in the arrestin-dependent pathway could proceed, further paving our understanding of this central process in cell-cell communication.